Our Services
HUMAN MEDICINAL PRODUCT DEVELOPMENT AND REGISTRATION
e.g. Development and validation of state-of-the-art in-vitro/in-vivo models
e.g. Performance of clinical phases I, II,III studies
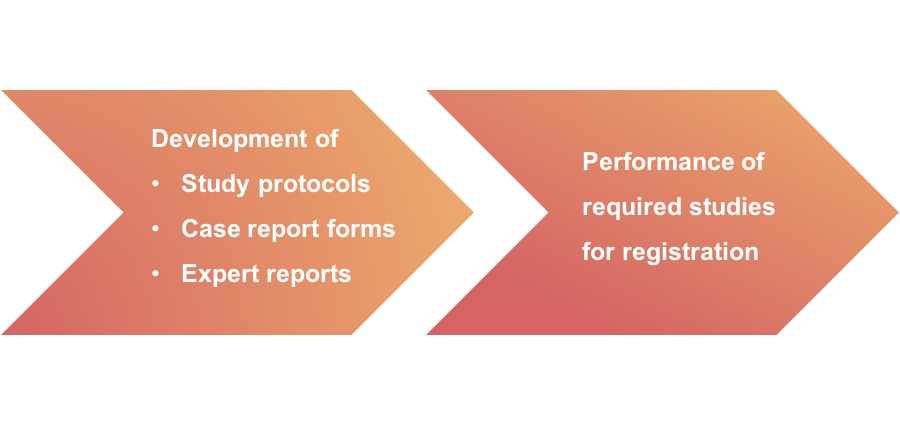
The process for the development and registration of human medicines has never been more complex or time-consuming. At IPSS, our aim is to make the development process as streamlined, efficient, and cost-effective as possible.
We have the know-how to ensure your company has the optimal development plan from study design and preparation of study documentation to the performance of the required studies. Naturally, all aspects of the study design and preparation are carried out according to ICH, GCP and GLP requirements.
INNOVATIVE GENERICS DEVELOPMENT
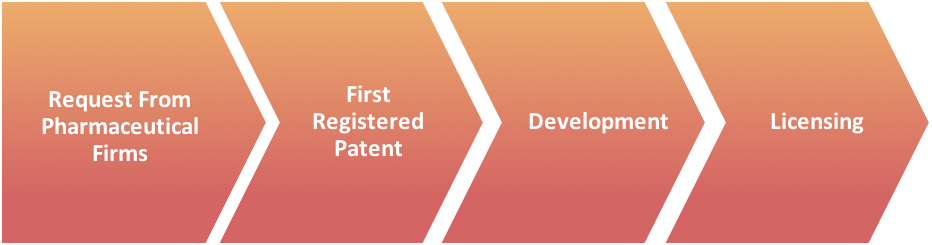
First Registered Patent For:
- Technology
- Therapeutic Indication
- Active Agents
- Drug Targeting Systems
- Synthesis
For those companies with ideas for novel generics we can provide a wide range of consultancy services to turn your innovation into tangible, focused, and comprehensive development strategies:
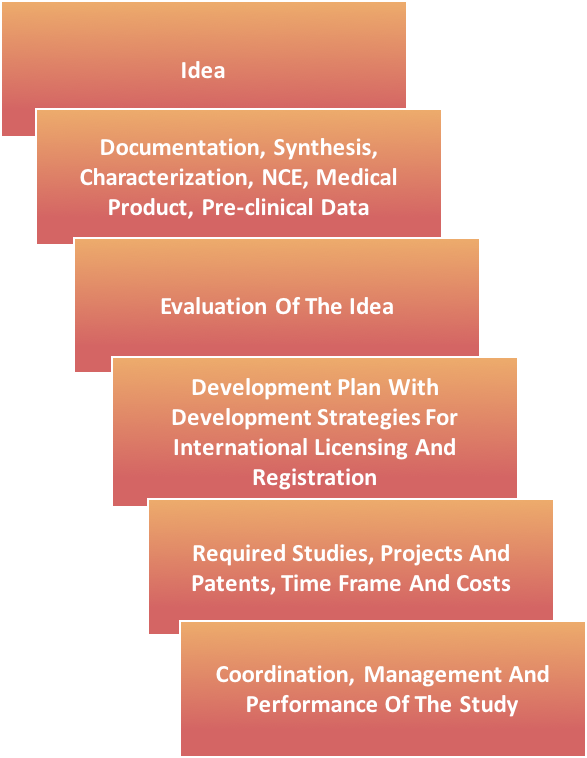
NEW CHEMICAL ENTITIES (NCE’S) DEVELOPMENTS
- Therapeutics
- Drug Delivery and Targeting Systems

- Studies will be performed with network of strategic partners.
- Development time for Pharmaceutical Analysis and Bioanalysis has a minimum of 10-24 months and a maximum of 36-48 months.
CONSULTING DRUG R&D
- Drug Discovery
- Preclinical Research
- Clinical Research
- Patent Strategy
- Quality Management Systems
